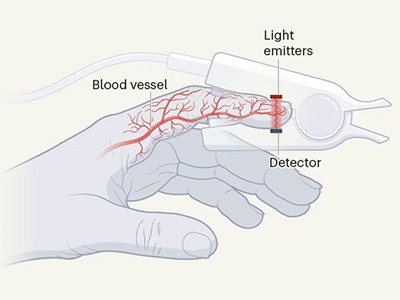
Pulse oximeters shine light through the skin and measure how much of it is absorbed by the blood to determine blood-oxygen levels.Credit: Francine Orr/Los Angeles Times via Getty
A physician in California is pursuing a lawsuit against 12 companies over their continued sale of devices that researchers say inaccurately measure blood-oxygen levels in people of colour. Studies1,2,3,4 — some decades old — have established that the devices, called pulse oximeters, can overestimate the amount of oxygen in the blood of people with dark skin, which could lead health professionals to delay or decide against treatment.
Skin colour affects the accuracy of medical oxygen sensors
The suit — filed by the Roots Community Health Center in Oakland, California, which is led by physician and researcher Noha Aboelata — is the first to take aim at the manufacturers of the devices. It asks for an injunction prohibiting further sales of the devices in California until they provide accurate readings for people of colour, or until warning labels are attached to note their inaccuracies.
Clipped over a fingertip, pulse oximeters determine a person’s blood-oxygen level by shining light through the skin and measuring its absorption by the blood. The measurement, considered one of a person’s ‘vital signs’, can give physicians and emergency responders quick insight into a person’s health. But high amounts of a type of melanin pigment in dark skin can interfere with the devices’ performance.
During the COVID-19 pandemic, in which people infected with the SARS-CoV-2 coronavirus sometimes had abnormally low oxygen levels, physicians put a spotlight on the problem2.
But researchers who spoke to Nature expressed disappointment in the slow pace of response from the medical-device industry and the US government. Legal experts say that this lawsuit offers an avenue to address the controversial devices that hasn’t been explored before, and even though it was filed in a California county court, could have ripple effects because of how large the state’s medical-device market is .
Is a racially-biased algorithm delaying health care for one million Black people?
“It feels awfully slow moving,” says Michael Sjoding, a pulmonologist at the University of Michigan in Ann Arbor who has conducted seminal research on pulse oximeters and race. “And there haven’t been any explicit changes I’ve heard from any companies.”
Nature contacted all of the companies named in the suit to seek their responses. The medical-device developer Medtronic, based in Minneapolis, Minnesota, says that it stands by its technology and has always “met or exceeded” all performance-testing guidelines set by the US Food and Drug Administration (FDA), which regulates medical devices.
Medical-device manufacturer Masimo, based in Irvine, California, points to a paper that it published in 20225 reporting that its oximeter found no significant difference between Black and white people when comparing oximeter readings against direct measurements of oxygen in blood samples. Theodore (Jack) Iwashyna, a physician and researcher at Johns Hopkins Medicine in Baltimore, Maryland, reviewed this study for Nature, however, and points out that Masimo measured healthy volunteers in a laboratory setting, rather than people who were ill in a real-world one. He maintains that pulse oximeters used in clinical settings can give inaccurate readings for people of colour, pointing to a recent systematic review.
Slow moving
The problem with the devices, say researchers who spoke to Nature, is that they were historically trained on white people. The biases have “been known about for a long time, but it didn’t really get any traction until COVID,” says Philip Bickler, an anaesthesiologist at the University of California, San Francisco, whose lab has been studying the devices since the 1980s. The turning point, he says, was a 2020 paper co-authored by Sjoding2. It showed that, in people with COVID-19, Black people were three times as likely as white people to receive pulse oximeter readings in a ‘safe’ range when, in fact, their blood-oxygen levels were dangerously low.

During the pandemic, Roots Community Health Center in Oakland, California, offered free COVID-19 tests at a walk-up site.Credit: Yalonda M. James/The San Francisco Chronicle via Getty
The study caught the attention of physicians, including Aboelata at Roots, a nonprofit organization that provides medical care mainly to people of colour. “When I discovered that this issue has been known by some researchers for decades,” Aboelata says, “it was frustrating.”
After the publication of that 2020 paper, several US senators urged the FDA to address the issue. The agency held a meeting of independent advisers in November 2022, which concluded that the devices gave less accurate readings for people of colour in real-world studies. The advisers also recommended funding two investigations, which Bickler is running, to assess pulse oximeters in a hospital setting. The committee will meet this February to assess the latest results.
“This is a complex issue, and the FDA continues to gather input from ongoing clinical research to help inform our decisions,” the agency told Nature, adding that it is exploring how to improve studies to evaluate oximeters’ performance before they are allowed on the market.
Meanwhile, Aboelata helped to survey the treatment that patients in a Northern California health-care system received. She and her colleagues confirmed that pulse oximeters overestimated blood-oxygen levels for Black people in that system — an effect that they showed correlated with these people receiving less care from physicians, or having to wait longer for it. After the team published the study in September 20224, Roots sent a letter to companies that manufacture or sell the devices, asking that they fix them or at least label them with a warning. “We were hopeful that manufacturers would want to do the right thing,” Aboelata says. Eventually, Roots filed suit, on 13 November 2023.
Hoping for action
Roots is invoking consumer-protection laws in California to argue that companies are falsely advertising the devices’ efficacy, when they might not be effective for people of colour. It names in its suit companies that manufacture the devices — including Medtronic and Masimo — as well as those that sell them directly to consumers, including the pharmacy chain CVS.

The first Indigenous female surgeon in Canada is battling for health justice
Researchers who spoke to Nature hope that the threat of a lawsuit will spur action in a way that publishing research papers has yet to do. “Up to now, a lot of it’s been a mix of denial and deflection — it’s been a problem,” Bickler says.
CVS responded to Nature’s queries saying that it couldn’t comment on pending litigation, but that “we’re committed to ensuring the products we offer work as intended, satisfy customers, and comply with all applicable laws, regulations and FDA guidance”. Medtronic says that it is “actively engaged with the FDA, academics, clinicians and patient advocates to continue to help reduce health disparities, and we hope to do the same with Roots Community Health Center”. Medical-equipment distributor Einstein Associates in Stafford, Texas, says that it “has not seen skin pigmentation playing a significant role in the accuracy of our pulse oximeters”.
Ripple effects?
Should the suit be heard before the court, the companies will probably argue that the FDA’s federal stamp of approval overrides state law, including the California consumer-protection laws that Roots is pointing to, says Carmel Shachar, a health-policy expert at Harvard University in Cambridge, Massachusetts. But it’s “an open question”, whether the FDA’s authority will hold, Shachar says.

‘There’s no space for us’: an Indigenous-health researcher battles racism in Australia
That’s because the FDA applies what is called the 510(k) pathway to approve pulse oximeters used by physicians. With this pathway, companies do not need to collect clinical-trial data for each new pulse oximeter, and can instead get the seal of approval by proving that their devices are “substantially equivalent” to one that is already on the market. Two federal cases brought before the US Supreme Court — both against Medtronic, one in 1996 and one in 2007 — ended with rulings suggesting that devices approved through the 510(k) pathway are not as well protected as some might think, Shachar says.
If Roots is successful with its case, it also remains to be seen whether the suit will have effects beyond California. Because of the size of its population, California’s “standards have a tendency to go beyond its border”, Shachar says. For example, the California Consumer Privacy Act sets stringent policies about the data that companies can collect about consumers. Although the law is in effect only in California, firms across the United States have developed websites that adhere to those policies. There could be similar trickle-down effects for pulse oximeters if Roots wins, says Sara Gerke, a legal expert at Penn State Dickinson Law in Carlisle, Pennsylvania.
The health-care industry needs to take responsibility for systemic inequities in health care, including pulse oximeters that don’t work properly for dark-skinned people, Aboelata says. “If we want to correct those, we’re going to have to do more than symbolic things,” she says. That includes going to court. “This is not an easy fix by any stretch of the imagination.”

Sarah Carter is a health and wellness expert residing in the UK. With a background in healthcare, she offers evidence-based advice on fitness, nutrition, and mental well-being, promoting healthier living for readers.








