- Author, Laura Goodwin
- Role, BBC Scotland innovation correspondent
A ground-breaking new treatment for sickle cell disease is being manufactured in Edinburgh.
In November, the UK became the first country to authorise a medical therapy that uses a revolutionary new gene-editing tool.
Scientists use it to identify faulty genes in a person’s blood so they can disable them.
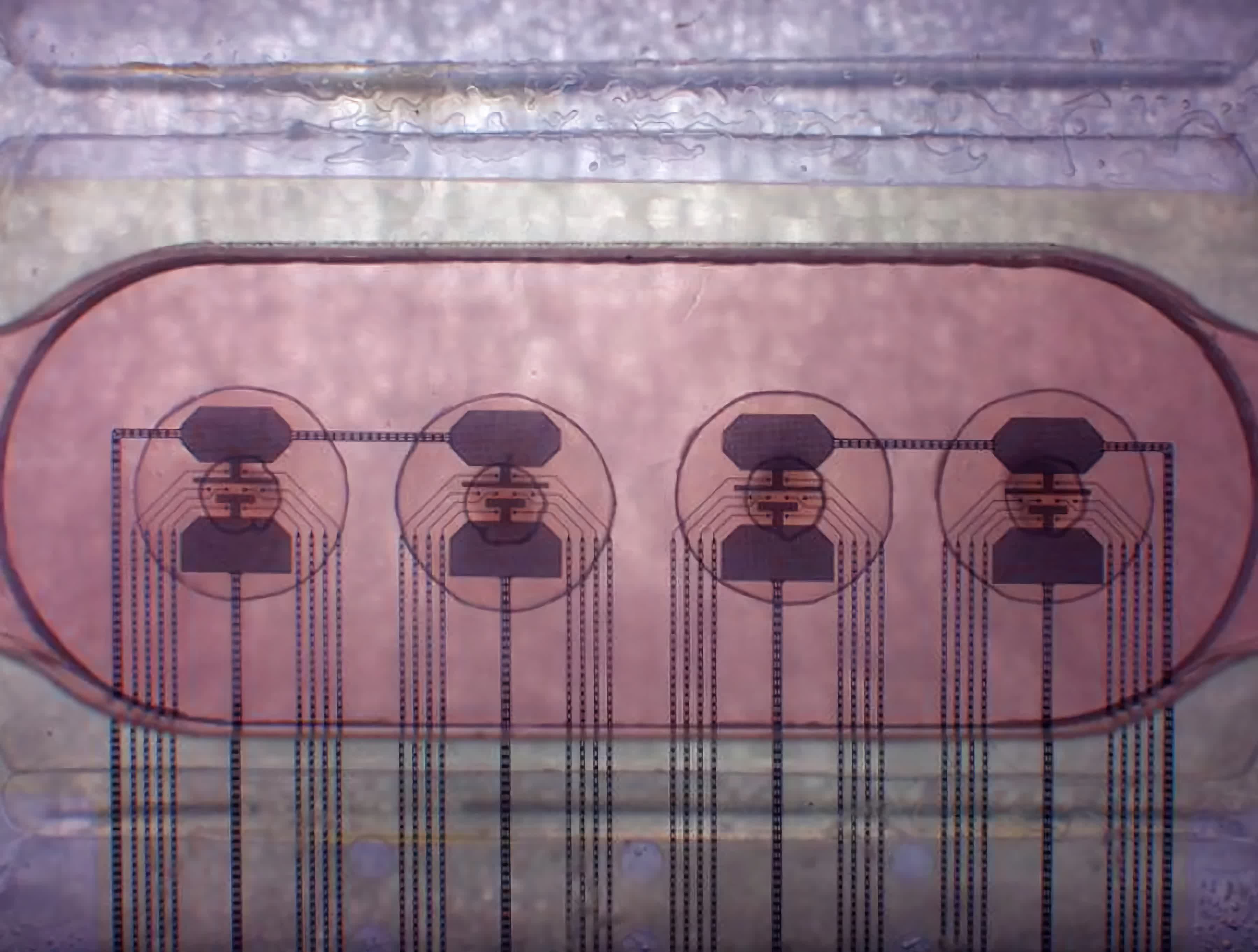
The RoslinCT lab, which specialises in cell and gene therapies, is helping to manufacture the therapy on behalf of US firm Vertex.
Its lab in Edinburgh’s bioquarter is now receiving samples from all over the world and wants to move to full commercial production of the therapy.
But there could be a major setback.
No price has yet been set for Casgevy, but the one-off treatment is likely to cost as much as £1.7m – which could be deemed too high a price for the NHS to bear.
It will now be up to assessment body Nice – the National Institute for Health and Care Excellence – to determine if it is cost-effective.
Sickle cell disease is an inherited blood disorder that affects haemoglobin, the protein red blood cells carries oxygen to the body.
Normal haemoglobin is disc-shaped and moves easily through blood vessels but sickle cells are crescent-shaped and can block blood vessels causing pain and damage to organs.
It can be life-threatening.
About 15,000 people in the UK have sickle cell disease, most with an African or Caribbean family background.
Almost 300 babies are born in the UK with sickle cell disease each year.
More than 1,000 people in the UK are affected by beta thalassemia, another disease that can be treated with new therapy.
Zaria Namutebi’s daughter has sickle cell
Zaria Namutebi’s nine year old daughter has sickle cell and the family spend a huge amount of time in hospital with her.
Zaria, originally from Uganda, wasn’t aware of any sickle cell disease in her family.
It was only through blood tests during her first pregnancy that she discovered she was a carrier of the sickle cell gene.
That means that although she doesn’t have the disease she can pass the gene on to her children.
Her daughter was tested through a heel prick test as a newborn and found to have sickle cell disease.
“She was well for the first year of her life and then all the things come in like the swelling of the fingers and the swelling of the toes,” Zaria says.
After that they “used to live in hospital”, she says.
“It felt like we were there all the time.”
It has changed almost every part of their lives.
Zaria is hopeful for a treatment for the condition.
Treatments of the future
A gene-editing treatment called Casgevy has been authorised by the MHRA and is being produced in Edinburgh.
Billions of bone marrow stem cells, which produce red blood cells, are harvested and their DNA is “edited” in the Edinburgh laboratory using a gene-editing technique called Crispr.
A single gene is disabled so the blood cells produce a substance known as foetal haemoglobin, which the body usually stops making in infancy and which reverses the sickle cell symptoms.
The modified cells are then injected into the body.
In trials, 28 out of 29 sickle cell patients were free of severe pain and 39 of 42 beta thalassemia patients no longer needed blood transfusions for at least a year.
It’s hoped it could be a permanent fix.
Peter Coleman from RoslinCT says these will be treatments of the future
Peter Coleman, from Roslin CT, said: “These will be the treatments of the future.
“They are not just tackling the symptoms, they are providing cures for individuals by genetically modifying the cells in people’s bodies.”
Rowan Flynn, the principal scientist at RoslinCT, said: “While Casgevy is specific for beta thalassaemia and sickle cell disease, the Crispr technology as a whole is extremely versatile and can be widely applied to other diseases that have some sort of genetic component to them.”
This is being hailed as big moment for gene therapy, but much is still unknown about this particular treatment
No price has yet been set for Casgevy in the UK.
A spokeswoman for Vertex said: “The US list price published for Casgevy is $2.2 m.
“We have not established a list price for the UK at this time. We are focused on working with the health authorities to secure reimbursement and access for eligible patients as quickly as possible.”
It will be up to assessors to determine if it is cost-effective, but it has brought hope.

Sarah Carter is a health and wellness expert residing in the UK. With a background in healthcare, she offers evidence-based advice on fitness, nutrition, and mental well-being, promoting healthier living for readers.