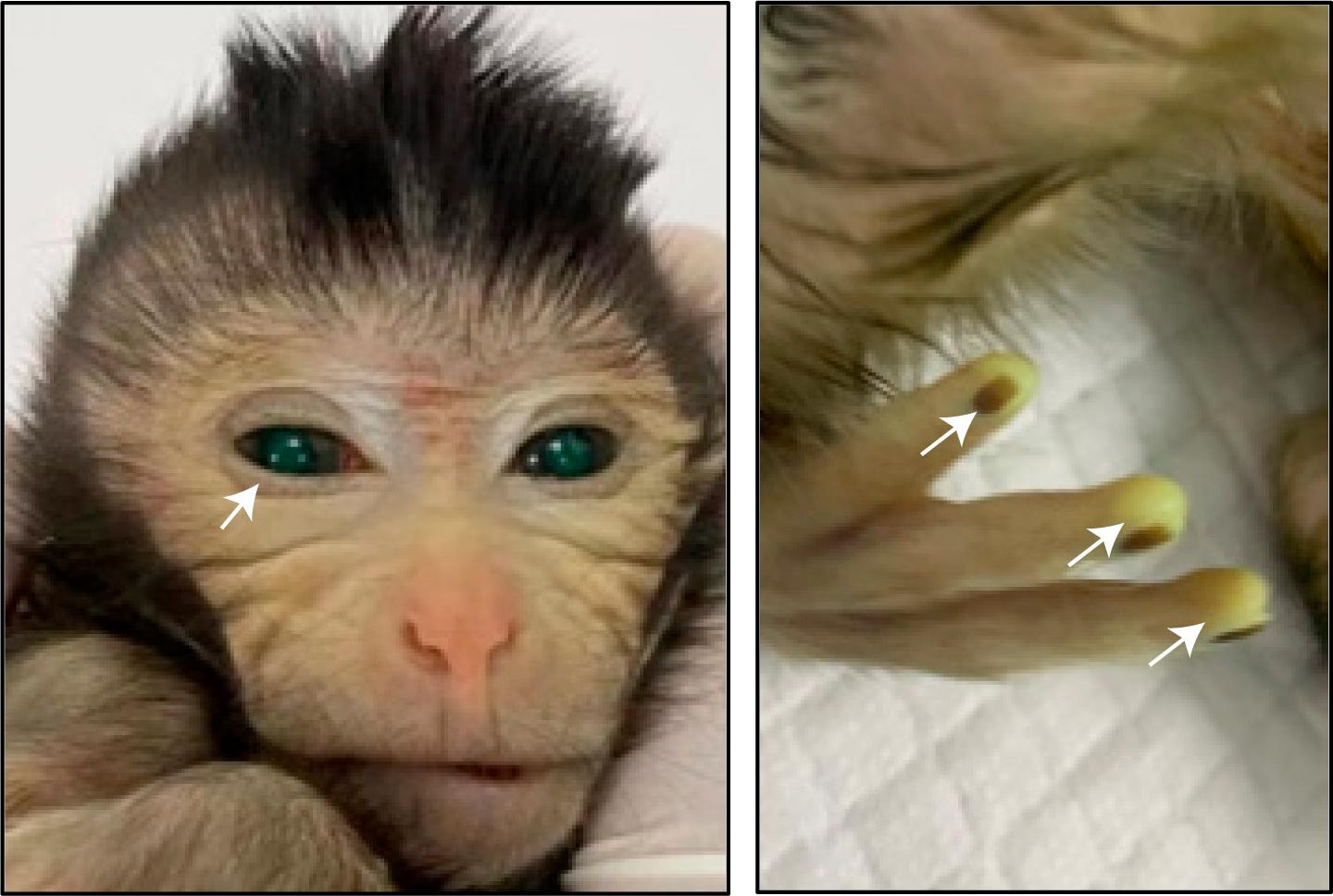
Images showing the green fluorescence signals in different body parts of the live-birth chimeric monkey at the age of 3 days Credit: Cell/Cao et al.
Breakthrough in Primate Research: Birth of a Chimeric Monkey
Chinese researchers have reported the first live birth of a chimeric monkey, a significant breakthrough in primate research. This achievement opens new avenues in understanding stem cell pluripotency and has significant implications for genetic engineering and biomedical studies.
A team of researchers in China has reported for the first time the live birth of a monkey that contains a high proportion of cells derived from a monkey stem cell line. This “chimeric” monkey is composed of cells that originate from two genetically distinct embryos of the same species of monkey. This has previously been demonstrated in rats and mice but, until now, has not been possible in other species, including non-human primates. The details of the research are reported November 9 in the journal Cell.
Implications in Pluripotency and Biomedical Research
“This is a long-sought goal in the field,” says senior author Zhen Liu of the Chinese Academy of Sciences (CAS). “This research not only has implications for understanding naive pluripotency in other primates, including humans, but it also has relevant practical implications for genetic engineering and species conservation. Specifically, this work could help us to generate more precise monkey models for studying neurological diseases as well as for other biomedicine studies.”
Methodology of the Study
The monkeys used in the study were cynomolgus monkeys, also known as crab-eating or long-tailed macaques, a primate common in biomedical research. The investigators first established nine stem cell lines using cells removed from 7-day-old blastocyst embryos. They then placed the cell lines in culture to give them enhanced ability to differentiate into different cell types.
They performed a number of different tests on the cells to confirm that they were pluripotent—having the ability to differentiate into all of the cell types needed to create a live animal. The stem cells were also labeled with green fluorescent protein so the researchers would be able to determine which tissues had grown out of the stem cells in any animals that developed and survived.
Successful Generation of Chimeric Monkeys
Ultimately, the scientists selected a particular subset of stem cells to inject into early monkey morula embryos (embryos that are 4–5 days old). The embryos were implanted into female macaques, resulting in 12 pregnancies and six live births.
An analysis confirmed that one monkey that was born alive and one fetus that was miscarried were substantially chimeric, containing cells that grew out of the stem cells throughout their bodies. Both were male. The investigators used the green fluorescent protein label to determine which tissues contained cells derived from the injected stem cells.
They also used gene sequencing and other tests to confirm the presence of stem-cell-derived tissue across different organs. The tissue types they tested that contained the stem-cell-derived cells included the brain, heart, kidney, liver, and gastrointestinal tract. In the live monkey, the contribution of the stem cells in the different tissue types ranged from 21% to 92%, with an average of 67% across the 26 different types of tissue that were tested. The numbers were lower in the monkey fetus.
In both animals, they also confirmed the presence of stem-cell-derived cells in the testes and in cells that eventually develop into sperm cells.
Future Directions and Enhancements
“In this study, we have provided strong evidence that naive monkey pluripotent stem cells possess the capability of differentiating in vivo into all the various tissues composing a monkey body,” says co-corresponding author Miguel Esteban of BGI Research and CAS. “This study deepens our understanding of the developmental potential of pluripotent stem cells in primate species.”
“This work helps us to better understand naive pluripotency in primate cells,” adds co-corresponding author Qiang Sun of CAS. “In the future, we will try to increase the efficiency of this method for generating chimeric monkeys by optimizing the culture conditions for the stem cells, the cultures for the blastocysts where the stem cells are inserted, or both.”
The investigators also plan to further explore the mechanisms that underlie the survival of the embryos in the host animals, which they say will help improve the efficiency of chimera generation.
Reference: “Live birth of chimeric monkey with high contribution from embryonic stem cells” by Jing Cao, Wenjuan Li, Jie Li, Md. Abdul Mazid, Chunyang Li, Yu Jiang, Wenqi Jia, Liang Wu, Zhaodi Liao, Shiyu Sun, Weixiang Song, Jiqiang Fu, Yan Wang, Yong Lu, Yuting Xu, Yanhong Nie, Xinyan Bian, Changshan Gao, Xiaotong Zhang, Liansheng Zhang, Shenshen Shang, Yunpan Li, Lixin Fu, Hao Liu, Junjian Lai, Yang Wang, Yue Yuan, Xin Jin, Yan Li, Chuanyu Liu, Yiwei Lai, Xuyang Shi, Patrick H. Maxwell, Xun Xu, Longqi Liu, Muming Poo, Xiaolong Wang, Qiang Sun, Miguel A. Esteban and Zhen Liu, 9 November 2023, Cell.
DOI: 10.1016/j.cell.2023.10.005
This work was funded by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Major Project, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Basic Frontier Scientific Research Program of Chinese Academy of Sciences, the National Science and Technology Innovation 2030 Major Program, and Shenzhen Basic Research Project for Excellent Young Scholars.

Dr. Thomas Hughes is a UK-based scientist and science communicator who makes complex topics accessible to readers. His articles explore breakthroughs in various scientific disciplines, from space exploration to cutting-edge research.